Insights
Immature Overall Survival Data in NICE Oncology Appraisals: Why It Matters for Decision-Making
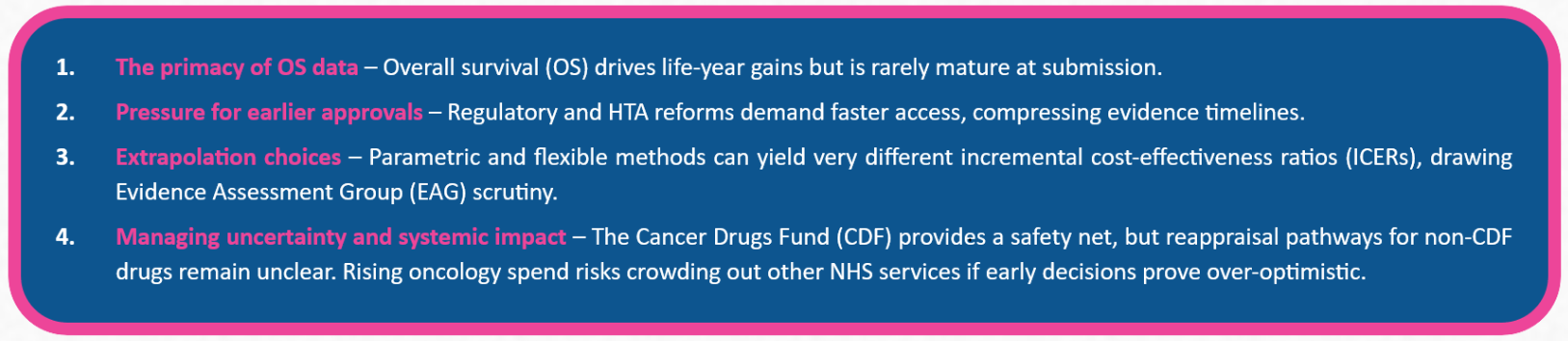
When making reimbursement decisions for new cancer treatments, health technology assessment (HTA) bodies like the National Institute for Health and Care Excellence (NICE) face a familiar challenge: how much trust should be placed in long-term survival predictions derived from short-term clinical data? In practice, the question of immature survival evidence manifests through four main challenges in NICE oncology appraisals.
1. Why OS matters
Within clinical trials, OS is generally defined as the time from randomisation until death from any cause. Although surrogate outcomes such as progression-free survival are widely used,1 OS remains the benchmark for assessing clinical benefit in oncology. Because NICE expects cost-effectiveness analyses to capture all meaningful differences in costs and outcomes between treatments, lifetime horizons are typically applied in oncology modelling.
2. Pressure for earlier approvals
To enable faster patient access, regulatory bodies often approve medicines during Phase 3 trials, and in some cases even after Phase 2, based on interim analyses rather than mature datasets. HTA agencies have mirrored this drive for speed, seen for example in the European Joint Clinical Assessment reforms and NICE’s proportionate approach to technology appraisals.2,3 As a result, OS evidence is frequently immature at the point of submission, requiring manufacturers to use statistical modelling to extrapolate survival beyond the observed trial period.
3. Extrapolation under scrutiny
Extrapolations are far from uniform. The modelling method selected can meaningfully affect projected survival and, in turn, the ICER. Standard parametric models apply a single distribution, while more flexible techniques (such as piecewise or spline-based approaches)may better reflect complex hazard functions but introduce extra assumptions anduncertainty.4 Because of this, extrapolation is a central focus of EAG critique, as NICE decisions made on immature data can have long-term implications for patient access, National Health Service resources, and the credibility of reimbursement outcomes.
4. Managing uncertainty: CDF and beyond
NICE has several ways of managing the uncertainty that comes with immature survival evidence. One option is to optimise recommendations by limiting use to patient groups where the benefit is most robust. In England, the CDF provides another route, granting earlier access to promising therapies while additional data are gathered. Under managed access, further data such as longer-term survival outcomes is collected, often leading to reappraisal within two years.5
For medicines that bypass the CDF, however, reappraisal procedures are less clearly defined, raising doubts about the durability of recommendations made on immature OS data. Projections from the OECD suggest cancer could add £14.4 billion to annual UK health spending by 2050, and some analyses indicate that the benefits of new treatments may not fully offset the opportunity costs of reduced investment in other services.6 In this context, approving medicines without safeguards like CDF entry risks locking in spending commitments that may later prove difficult to justify.
Symmetron’s research
With OS playing such a critical role in oncology submissions, we set out to explore the characteristics of NICE oncology STAs that still achieved a full recommendation despite immature survival evidence. To answer this question, we systematically reviewed all oncology technology appraisals published over a five-year period. We included only those with a positive recommendation where OS data were deemed immature based on EAG feedback at the time of submission and excluded appraisals with optimised recommendations or those routed through the CDF.
This blog post is part of a series, here in Part 1 we focused on the methodological context, whilst Part 2 will explore the characteristics of these appraisals. If you are navigating similar challenges in preparing evidence for a NICE oncology submission, Symmetron can support with modelling strategy, evidence generation, and HTA engagement. We’d be pleased to discuss how we can help.
References
1. Beauchemin C, Johnston JB, Lapierre MÈ, Aissa F, Lachaine J. 2025. Relationship between progression-free survival and overall survival in chronic lymphocytic leukemia: a literature-based analysis. Curr Oncol. 22(3):e148-56.
2. European Commission. Joint clinical assessments. 2024. Available: https://health.ec.europa.eu/health-technology-assessment/implementation-regulation-health-technology-assessment/joint-clinical-assessments_en
3. Association of the British Pharmaceutical Industry (ABPI). 2023. NICE’s proportionate approach to technology appraisals: a guide for pharmaceutical companies. Available: https://www.abpi.org.uk/media/kktgeaov/nice-proportionate-approach-to-technology-appraisals-18102023.pdf
4. Latimer NR, Adler AI. 2022. Extrapolation beyond the end of trials to estimate long term survival and cost effectiveness. BMJ Med, 1:e000094.
5. National Institute for Health and Care Excellence (NICE). 2025. Managed access. Available: https://www.nice.org.uk/what-nice-does/patient-access-schemes-and-pricing-agreements/managed-access
6. Naci H, et al. 2025. Population-health impact of new drugs recommended by the National Institute for Health and Care Excellence in England during 2000–20: a retrospective analysis. Lancet. 405(10472):50-60.
Similar Insights
Stay in touch
Subscribe to Symmetron and stay up to date with recent news and announcements.






.jpg)




















