Insights

When “Immature” Doesn’t Mean “Unusable”: What NICE’s Oncology Decisions Reveal About OS Evidence
For anyone developing oncology treatments today, one challenge looms large: overall survival (OS) data rarely mature fast enough to keep pace with innovation. Yet market access cannot wait years for long-term follow-up — and, crucially, NICE doesn’t always expect it to.
Over a five-year period of oncology technology appraisals, more than half of NICE’s fully recommended treatments were approved despite immature OS evidence at submission. This tells an important story: NICE is less interested in whether OS is “finished,” and far more interested in whether OS benefit is credible.
So what does “credible” look like — and what can manufacturers learn from the pattern?
What the data show: immature OS is common and often accepted
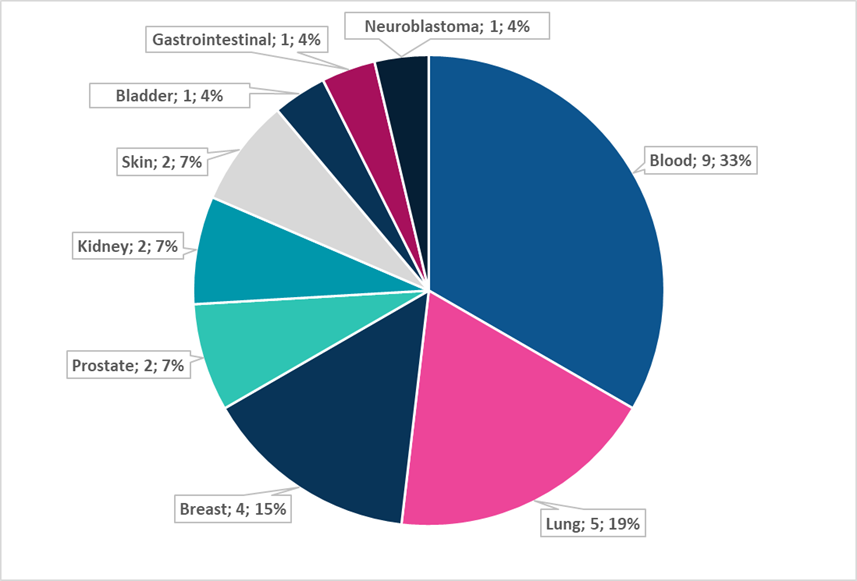
Between 2018 and 2022, NICE reviewed 118 cancer single technology appraisals. Forty-nine received a full recommendation, and 27 of these relied on immature OS.
These submissions covered a wide range of modalities, from tyrosine kinase inhibitors and checkpoint inhibitors to monoclonal antibodies, hormonal therapies, and more. Tumour types were equally diverse, from haematological cancers to lung, breast, prostate, kidney, and others.
In other words: the challenge is universal, and so is the path forward.
Figure 1. Disease distribution of NICE oncology positive recommendations based on immature OS data
How companies approached OS and how NICE responded
No single modelling strategy dominated. Submissions used everything from standard parametric models to surrogate-based estimation, mixture-cure approaches, piecewise models, or — in one case — no OS modelled at all. The exponential model was most common, while six appraisals leaned on PFS-based surrogate approaches.
Table 1. Company base-case OS modelling strategies used in recommended NICE oncology appraisals with immature OS data
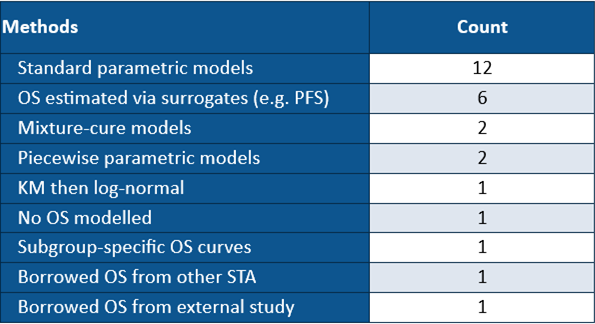
NICE’s Evidence Assessment Groups (EAGs) accepted about a third of the base-case extrapolations outright; in the rest, they recommended alternatives.
This widespread illustrates a crucial truth: there is no “safe” template for OS modelling when the data are immature — but there are consistent signals of what NICE may find persuasive.
Three patterns behind NICE’s willingness to accept immature OS
1. Strong clinical plausibility anchors the case
Clinical and Cancer Drugs Fund (CDF) expert judgement consistently influenced decisions. When experts confirmed that OS benefit was biologically plausible, whether due to class effects, mechanistic rationale, or observed patterns such as PFS advantage, NICE was willing to accept an assumed survival benefit even without long-term proof.

2. Surrogates matter — especially in the adjuvant setting
In adjuvant oncology, where survival takes years to manifest, NICE routinely accepted endpoints such as recurrence-free or relapse-based outcomes as meaningful indicators of eventual OS.
When prognosis improves by preventing recurrence, the long-horizon OS impact becomes a reasonable assumption — even if the data cannot yet show it.

3. Transparent management of uncertainty goes further than confidence intervals
NICE showed willingness to accept OS uncertainty when companies demonstrated they had bounded it responsibly — through conservative scenarios, robust sensitivity analyses, and plausible alternative curves.
Importantly, strong OS signals that were trending towards statistical significance also contributed to positive decisions when aligned with mechanistic rationale and other supportive endpoints.

What this means for pharmaceutical companies preparing NICE submissions
The takeaway is not simply that immature OS can be accepted. It’s that NICE rewards coherent narratives, grounded in:
- clinical plausibility,
- biologically aligned surrogates,
- transparent modelling choices, and
- honest reflection of uncertainty.
Based on our recent research, positive recommendations were still made when immature OS data were supported by a coherent clinical narrative and transparent modelling, suggesting that credible evidence can offset gaps in maturity.
How can Symmetron help
Preparing submissions with immature OS evidence requires a blend of strategic modelling, evidence generation, and HTA-aware story telling. Symmetron specialises in shaping these arguments so they resonate with clinical experts, EAGs, and decision committees alike.
If you’re navigating similar challenges, we’d be pleased to discuss how we can support your next NICE submission.
Similar Insights
Stay in touch
Subscribe to Symmetron and stay up to date with recent news and announcements.





.jpg)




















